Polymer Based Nanomaterials
Subtopics: Macromolecular amphiphiles / Polymer Synthesis / Diblock Copolymers / Triblock Terpolymers / Extended Amphiphilic Dendrons / Thermoresponsive Hydrogel Mobility / Complex Fluids Under Shear
Macromolecular amphiphiles
The terms macromolecule and polymer refer to large molecules whose structure depends on the monomer or monomers used in their preparation. A polymer prepared from a single monomer is called a homopolymer. If two or more monomers are employed, the product is a copolymer. In copolymers the monomeric units may be distributed randomly (random copolymer), in alternating fashion (alternating copolymer), or in blocks (block copolymer).
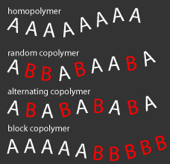
If the backbone consists of a single block of each monomer A and B, the polymer is an AB diblock copolymer. Other possibilities include ABA and ABC triblock copolymers. One can also describe polymers by their connectivity as linear, branched or network.

An amphiphile is a molecule that possesses both a hydrophilic and a hydrophobic nature. Soaps, fatty acids and some block copolymers are examples of amphiphilic molecules. It is common for amphiphiles to spontaneously self-assemble into a diversity of morphologies. We use this property of macromolecular amphiphiles to design hybrid materials.
Polymer Synthesis
Our group synthesizes block copolymers in order to direct the structure of hybrid materials and to study complex fluids. Most of our polymerization reactions are done through either anionic polymerization or living radical polymerization.
- Anionic Polymerization
The most widely used technique for the preparation of model block copolymers with homogeneous chain length is living anionic polymerization. The active centers in anionic polymerization processes are highly reactive carbanions.
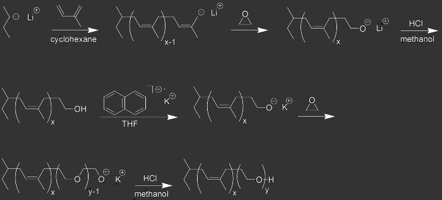
Because they are very sensitive to oxygen and acidic protons (water, alcohols etc.) it is necessary to exclude any impurities. This can be achieved by using vacuum or working in inert atmosphere (glovebox). Our group is equipped with four high vacuum lines and two glove boxes which are used for polymerizations of various monomer liquids and gases. Some typical polymers made by anionic polymerization are polyisoprene, polystyrene, poly(ethylene oxide) and block copolymers like poly(styrene-block-isoprene), PS-b-PI, poly(isoprene-block-ethylene oxide), PI-b-PEO and poly(isoprene-block-N,N-dimethylaminoethyl methacrylate), PI-b-PDMAEMA.
- Atomic Transfer Living Radical Polymerization (ATRP)
Another commonly used technique for block copolymer preparation is ATRP. An equilibrium reaction between an alkyl halide (P-X) and a transition metal (Mt) creates a low concentration of radicals to enable polymerization (+M) while limiting unwanted termination reactions.
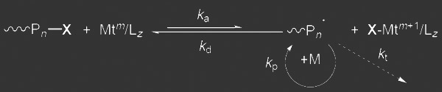
ATRP is both versatile and robust in that it can polymerize most vinyl monomers and is tolerant of water as well as many side groups. ATRP has been used to create block copolymers such as poly(ethylene oxide-block-acrylonitrile), PEO-b-PAN.
- Combination of Anionic and ATRP Techniques
The combination of anionic polymerization and controlled radical polymerization of vinyl monomers allows one to prepare copolymer structures that are difficult to achieve through pure sequential anionic polymerization. Well-defined polymeric architectures have been engineered via this novel route. Our group has synthesized "frustrated" amphiphilic triblock terpolymers by combining anionic polymerization and ATRP. More details on this work may be found on our Triblock terpolymers section.
Diblock Copolymers
The unlike blocks in block copolymers tend to phase separate into microdomains forming different mesophases. The size scale of the domains is governed by the chain dimensions, while the block ratio determines the mesophase structure. The known equilibrium mesophases for diblock copolymers, which include spheres (S), cylinders (C), gyroid (G), and lamellae (L) are shown in the phase diagram below.
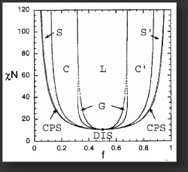

Our group is involved in the synthesis and characterization of functional polymers with different molecular architectures. Fundamental studies on AB block copolymer thermodynamics rely on the preparation of monodispersed materials with well-defined molecular weights (MWs) and block volume fractions (f).
References:
[1] S. Mahajan, S. Renker, P. Simon, J. Gutmann, A. Jain, S. Gruner, L. Fetters, G. Coates, U. Wiesner, "Synthesis and characterization of amphiphilic poly(ethylene oxide)-block-poly(hexyl methacrylate) copolymers", Macromolecular Chemistry and Physics 204 (8), 1047-1055 (2003).
[2] P. Gopalan, Y. Zhang, X. Li, U. Wiesner, C. K. Ober, "Liquid Crystalline Rod-Coil Block Copolymers by Stable Free Radical Polymerization: Synthesis, morphology, and rheology", Macromolecules 36, 3357-3364 (2003).
[3] M. Schoeps, H. Leist, A. DuChesne, U. Wiesner, "Salt-Induced Switching of Microdomain Morphology of Ionically Functionalized Diblock Copolymers", Macromolecules 32, 2806-2809 (1999).
[4] V.Schaedler, J. Spickermann, H.-J. Raeder, U. Wiesner, "Synthesis and Characterization ofa,w-Zwitterionic Block copolymers of Styrene and Isoprene", Macromolecules 29, 4865-4870 (1996).
Triblock Terpolymers
Triblock terpolymers are a natural extension of our work with diblock copolymers. Tri-block terpolymers may be thought of simply as a diblock copolymer (AB) upon which a third block (C) is grown.
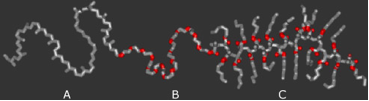
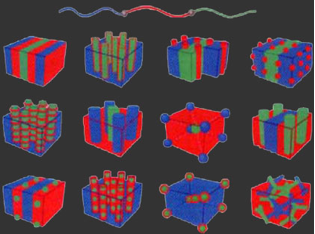
Twelve possible triblock terpolymer mophologies are shown in the figure below, including some core-shell analogs of the well-known diblock phases. Of particular interest in triblock terpolymers is the large phase space over which continuous network morphologies are observed, opening the door to many new and exciting applications of mesostructured materials. Triblock copolymers have more experimental parameters than diblock copolymers. For a given triblock copolymer system, there are three different Flory-Huggins interaction parameters χ_AB, χ_BC and χ_AC. Furthermore, for each particular polymer there are three additional independent parameters: the volume fraction of the A block (f_A), the volume fraction of the B block (f_B), and the degree of polymerization (N). Altogether, there are six parameters that determine the equilibrium structure of a given triblock terpolymer.
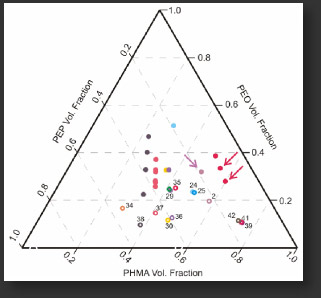
We have explored the phase space of the ABC polymer as shown in the ternary phase diagram below. Characteristic triblock terpolymer morphologies were observed including spherical, tubular and lamellar morphologies as shown below. In 2004, we reported the first triblock terpolymer ever to have a poly(ethylene oxide) (PEO) middle block.
References:
[1] S. Mahajan, B-K. Cho, J. Allgaier, L. Fetters, G. Coates, U. Wiesner. "Synthesis of amphiphilic ABC triblock copolymers with PEO as the middle block" Macromolecular Rapid Communications 25 (22), 1889-1894 (2004).
[2] Triblock morphologies figure adapted from: F. Bates, G. Fredrickson, "Block copolymers - designer soft materials", Physics Today 52 (2), 32-38 (1999).
Extended Amphiphilic Dendrons
An extension of linear-linear block-copolymers is the use of a linear polymer chain which is end-functionalized with a dendritic molecule. This molecular architecture is called a coil-dendron system. Dendrimers and dendrons constitute a subclass of dendritic structures and consist of
- a core
- an interior of shells consisting of repetitive branch-cell units
- terminal groups at the periphery
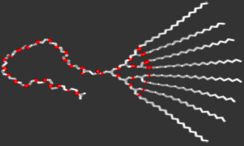
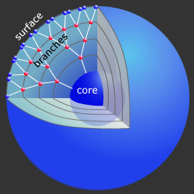
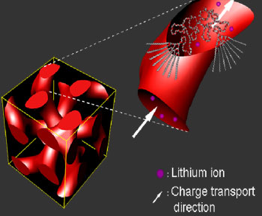
In our work, a hydrophobic poly(ether)-type dendron was extended with a linear hydrophilic polymer chain. These macromolecules self-assembled into micellar, 1D cylindrical, 2D lamellar and 3D continuous cubic mesophases. Charge transport within this nanostructured material was revealed upon doping the hydrophilic part with lithium ions, i.e. ion conductivity was confined to micelles, cylinders, lamellae or continuous throughout the network. This approach is of particular interest in areas where charge transport within polymeric matrices is becoming increasingly important, such as ion conductors, photovoltaic or electroluminescent cells.
References:
[1] B-K. Cho, A. Jain, J. Nieberle, S. Mahajan, U. Wiesner, S. Gruner, S. Tuerk, H. Raeder, "Synthesis and Self-Assembly of Amphiphilic Dendrimers Based on Aliphatic Polyether-Type Dendritic Cores" Macromolecules 37 (11) 4227-4234 (2004).
[2] B-K. Cho, A. Jain, S. Gruner, U. Wiesner, "Mesophase Structure-Mechanical and Ionic Transport Correlations in Extended Amphiphilic Dendrons", Science 305 (5690), 1598-1601 (2004).
Thermoresponsive Hydrogel Mobility
Motion at any scale attracts attention and interest. Several mechanisms for the generation of motion exist in nature on a wide range of length scales: from a macroscopic scale on which a snake crawls over a rough surface to a micro-scale where molecular motor proteins walk on microtubules. It is desirable to mimic these natural systems to make efficient devices for useful applications. While a biological approach is limited by the working environment of the device, a synthetic approach gives freedom to tune parameters as per the requirement. We take a synthetic approach to solve this problem and work with thermoresponsive hydrogels.
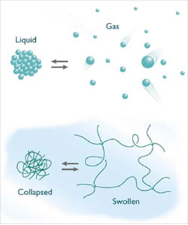
Hydrogels are a class of crosslinked polymers that can hold large volumes of water. Some of these polymer gels are highly sensitive to their environment and release their water upon small environmental changes. This is known as volume phase transition (VPT) and is similar to gas-liquid phase transition. We use a special kind of hydrogel, a hybrid hydrogel, in which nanocomposite clay particles are used as a crosslinker and N-Isopropylacrylamide is used as the monomer to form polymer chains between the crosslink points. The clay particles give the hydrogel robustness and the monomer makes the gel thermoresponsive. This hydrogel shows a Volume Phase Transition (VPT) at around 32 C which means when heated above 32 C the gel releases all its water. Since the process is reversible, the gel regains its size upon cooling.
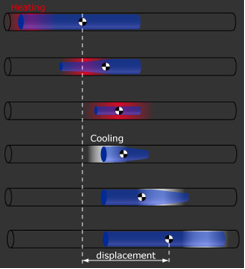
These gels move in a direction when VPT is asymmetrically induced along the length of the cylindrical gel, as shown below. To achieve this, we make long cylindrical gels inside a glass capillary and then raise temperature locally using a Peltier Element device, shown below. This device has 24 Peltier Elements which are individually controlled with a switch. Each element heats/cools a small section of the capillary from the bottom. We can move these gels several times in a millimeter diameter capillary, see below, with velocities ~ 1 um/sec. In comparison, one of the fastest crawling eukaryotes, Amoebae of Acrasis (with cell surface area of 759 m^2), moves with an average speed of 71.6 mm/min.
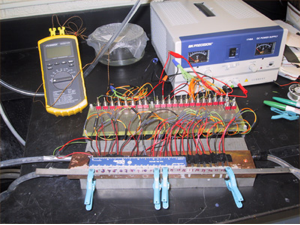
We have also shown that this device is capable of doing work by attaching a glass bead at the end of the gel and showing that the gel carries the bead with it. We anticipate that such devices can be widely utilized in a variety of areas in biotechnology including microfluidics, small-scale robotics and drug delivery.
References:
[1] L. Yeghiazarian, H. Arora, V. Nistor, C. Montemagno, U. Wiesner. "Teaching hydrogels how to move like an earthworm" Soft Matter 3, 939-944 (2007).
[2] L. Yeghiazarian, S. Mahajan, C. Montemagno, C. Cohen, U. Wiesner. "Directed Motion and Cargo Transport Through Propagation of Polymer-Gel Volume Phase Transitions", Advanced Materials 17 (15), 1869-1873 (2005).
[3] K. Haraguchi, H. Li, K. Matsuda, T. Takehisa, E. Elliot. "Mechanism of Forming Organic/Inorganic Network Structures during In-situ Free-Radical Polymerization in PNIPA-Clay Nanocomposite Hydrogels" Macromolecules 38, 3482-3490 (2005).
Complex Fluids Under Shear
Morphology processing correlations in complex polymer systems play a key role in various advanced materials, such as thermoplastic elastomers. Traditional manufacturing processes often lead to polydisperse morphologies of these materials. Precise control over microstructure is important even for conventional applications because mechanical properties such as impact strength and damping coefficient depend strongly on morphology. In addition, a large number of emerging novel applications of these materials across manyfields require the production of highly ordered, defect-free materials, these including:
- Sub-micrometer electronic circuits
- DNA electrophoresis media
- High density magnetic recording devices
- Filters with nanometer pore sizes
- Creation of quantum confinement for light emission
The ability to manipulate and control the microstructure is essential for these applications. Our primary research interest is exploring physical mechanisms underlying the behavior of self-assembled complex polymeric materials under external mechanical fields. The understanding of these mechanisms will eventually lead to invention of novel processing techniques of the materials. Experimental techniques explored:
- Rheology
- Small Angle X-Ray Scattering (SAXS)
- Transmission Electron Microscopy (TEM)
- In-Situ Structural Probe
In addition to using polymers as structure directing agents, we have investigated their rheological properties and used their volume phase transition to develop active polymeric materials. In conjunction with our block-copolymer structure direction work, we have sought to understand large-scale ordering of block copolymer domains through shear flow and thus desired to understand the rheological behavior of the polymers in question.
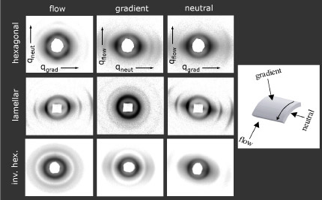
Using shear flow, we have developed a straightforward method for the macroscopic alignment of bulk polymer-nanoparticle hybrid materials from solutions. 2D SAXS patterns show orientation effects for hexagonal, lamellar and inverse hexagonal samples.
References:
[1] A. Jain, L. M. Hall, C. B. W. Garcia, S. M. Gruner, U. Wiesner, "Flow induced alignment of block copolymer directed organic-inorganic nanostructured silica-type hybrids", Macromolecules 38, 10095-10100 (2005).
[2] M. Langela, U. Wiesner, H. W. Spiess, M. Wilhelm, "Microphase reorientation in Block copolymer melts as detected via FT Rheology and 2D SAXS", Macromolecules 35, 3198-3204 (2002).
[3] U. Wiesner, "Flow alignment of block copolymers" in: The Encyclopedia of Science and Technology, Pergamon, An Imprint of Elsevier Science, Oxford, UK 2001
[4] H. Leist, D. Maring. T. Thurn-Albrecht, U. Wiesner, "Double Flip of Orientation for a Lamellar diblock copolymer under Shear", J. Chem. Phys. 110, 8225-8228 (1999).
[5] U. Wiesner, "Feature Article: Block copolymers under large amplitude oscillatory shear flow: order and dynamics", Macromol. Chem. Phys. 198, 3319-3352 (1997).
[6] Y. Zhang, U. Wiesner, Y. Yang, T. Pakula, H. W. Spiess, "Annealing Effects on Orientation in Dynamically Sheared Diblock.Copolymers", Macromolecules 29, 5427-5431 (1996).
[7] Y. Zhang, U. Wiesner, H. W. Spiess, "Frequency Dependence of Orientation in Dynamically Sheared Diblock Copolymers", Macromolecules 28, 778-781 (1995).
[8] Suggested Readings: Rolald G. Larson, The Structure and Rheology of Complex Fluids, Oxford University Press, 1999 Ian W. Hamley, The Physics of Block Copolymers, Oxford University Press, 1998