Silica Nanoparticles as Biological Probes
1. FDA approved C Dot (Cornell Dot) <-- Click this to learn more about C Dots
In collaboration with the Memorial Sloan Kettering Cancer Center (MSKCC) we demonstrated that small C dots that have been surface-modified with poly(ethylene glycol) (PEG) have efficient renal clearance in mice compared to unmodified C dots.
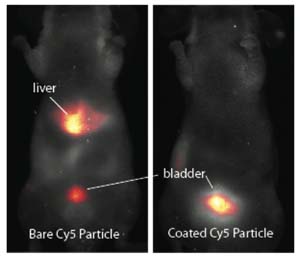
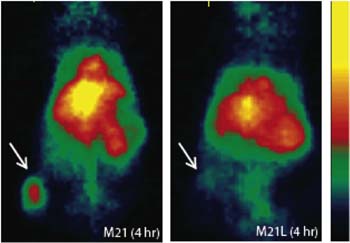
Further work using C dots that had been modified with the integrin-targeting peptide cRGDY, and radiolabeled with 124I showed that C dots can be used for selective tumor targeting and real-time multimodal imaging in both small and large animal models. This work has led to C dots receiving the FDA investigational new drug approval for a first-in-human clinical trial.
References:
[1] A. Burns, J. Vider, H. Ow, E. Herz, O. Penate-Medina, M. Baumgart, S. Larson, U. Wiesner, M. Bradbury. "Fluorescent Silica Nanoparticles with Efficient Urinary Excretion for Nanomedicine", Nano Lett. 9(1), 442-448 (2009).
[2] M. Benezra, O. Penate-Medina, P. Zanzonico, D. Schaer, H. Ow, A. Burns, E. DeStanchina, V. Longo, E. Herz, S. Iyer, J. Wolchock, S. Larson, U. Wiesner, M. Bradbury. "Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma", The Journal of Clinical Investigation 121(7), 2768-2780 (2011).
2. Biological Probes
The core-shell architecture of the C dot is ideally suited for the development of multifunctional probes for biology.
In collaboration with the Baird Group here at Cornell, we have demonstrated antibody-mediated labeling with Rat Basophilic Leukemia Mast Cells using the FCeRI cell surface receptor for IgE as shown below.
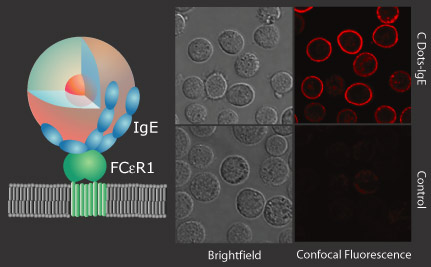
In addition to labeling, we have developed a class of sensor particles based on the core-shell C Dot architecture, known as C Dot sensors.
These particles use the core dye as an internal reference, allowing quantitative concentration measurements to be performed.
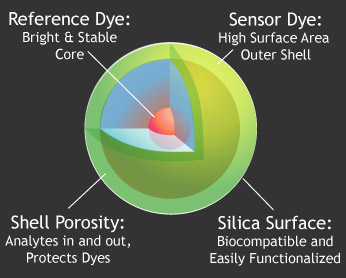
By placing the sensor dye in the surface shell, the maximum possible surface area is exposed for interaction with the environment
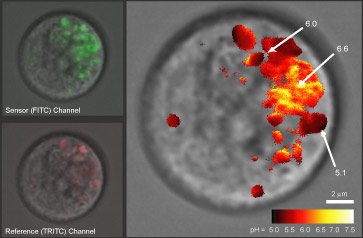
We have demonstrated ratiometric pH sensing in RBL cells using a FITC/TRITC labeled particle, as shown in the confocal images above. The individual reference and sensor signals were collected, compared for each pixel and analyzed to yield the false-spectrum image above showing the pH in various intracellular vesicles in a mast cell.
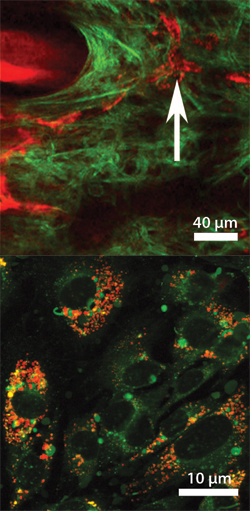
In vivo probes for Nanomedicine: Further, we have begun to study the biodistribution and biocompatibility of these nanoparticles in living systems. This work has shown the particles to be non-toxic in mice, and we have demonstrated in vivo imaging of the particles both in the bloodsteram as well as within cells. The images above show 30nm particles in the bloodstream via multiphoton microscpy (top, red), and spontaneously endocytosed by mouse prostate carcinoma cells via TAT cell-penetrating peptides conjugated to the particle surface (bottom, red).
References:
[1] J. Choi, A. Burns, R. Williams, Z. Zhou, A. Flesken-Nikitin, W. Zipfel, U. Wiesner, A. Niktin, "Core-shell silica nanoparticles as fluorescent labels for nanomedicine", Journal of Biomedical Optics 12 (6), 064007-(1-11) (2007).
[2] A. Burns H. Ow, U. Wiesner. "Fluorescent core-shell silica nanoparticles: towards 'Lab on a particle' architectures for nanobiotechnology" Chemical Society Reviews, 35, 2006, 1028-1042 A. Burns, P. Sengupta, T. Zedayko, B. Baird, U. Wiesner, "Core-Shell Fluorescent Silica Nanoparticles for Chemical Sensing: Towards Single Particle Laboratories", Small 2 (6), 723-726 (2006).
[3] E. Herz, A. Burns, S. Lee, P. Sengupta, D. Bonner, H. Ow, C. Liddell, B. Baird, U. Wiesner, " Fluorescent core-shell silica nanoparticles: an alternative radiative materials platform", Proceedings of the SPIE Vol. 6096: Colloidal Quantum Dots for Biomedical Applications, 1-12 (2006).
[4] H. Ow, D. Larson, M. Srivastava, B. Baird, W. Webb, U. Wiesner,"Bright and Stable Core-Shell Fluorescent Silica Nanoparticles", Nano Lett. 5 (1), 113-117 (2005).