Energy materials
Using block copolymer self-assembly to structure-direct inorganic materials is a major thrust of our group. For energy applications, control of functionality and performance is dictated by material choice and nanostructure. For example, high interfacial surface areas with controllable mesoscale pore size distributions are desirable in almost every energy conversion and storage device. Bottom-up self-assembly provides a low-cost approach to large area nanostructure fabrication and inherently allows 3D structure formation, in contrast to top-down techniques.
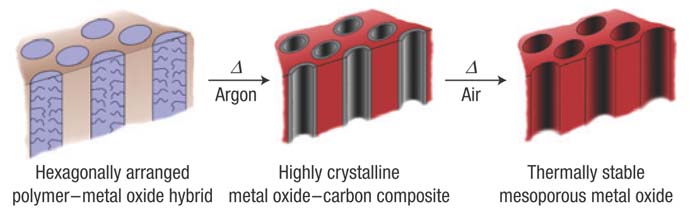
We have demonstrated a versatile approach using the Combined Assembly by Soft and Hard chemistries (CASH) method to structure-direct transition metal oxides allowing the nanostructures to survive in high temperature treatments [1]. Thus far, we have synthesized many different energy materials using the CASH method, including fuel cell electrodes, titanium dioxide (titania) electrodes for dye-sensitized solar cells and titania/carbon composite anode materials for lithium ion batteries.
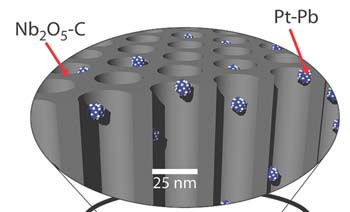
Stable conductive porous catalyst supports are needed to improve performance and reduce costs in fuel cells. More specifically, (1) conductivity is required for the electron transport in the redox reactions catalyzed by finely-dispersed noble metal nanoparticles; (2) porosity is important for fuel delivery; and (3) stability is needed to prevent support breakdown and subsequent loss of catalyst surface area. In our 2009 JACS paper, we demonstrated a one-pot synthesis of platinum-based nanoparticles incorporated into mesoporous niobium oxide-carbon composites, exhibiting majorly improved performance in formic acid electro-oxidation [2]. This approach shows a simple synthesis of a highly functional material built from block copolymer-directed self-assembly and can provide a major enhancement in electrodes research for polymer electrolyte membrane fuel cells.

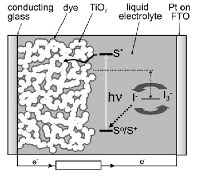
For dye-sensitized solar cells (DSSCs), the combination of high surface area and good connectivity of the semiconducting titania is desired to maximize dye absorption and electron transport, respectively. In our 2009 Nano Letters paper, we demonstrated the first successful application of an ordered bicontinuous gyroid semiconducting network in a solid-state DSSC with our collaborators at the Universities of Cambridge and Oxford [3]. The freestanding gyroid network is fabricated by electrochemical deposition into the 10 nm wide voided channels of a self-assembled, selectively degradable block copolymer template. More recently, we have also shown liquid-state DSSC devices using block-copolymer-directed titania electrodes with power conversion efficiencies up to 6.44% [4,5].
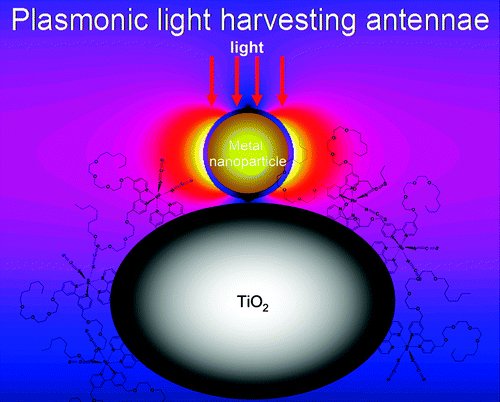
Furthermore, we demonstrate plasmon-enhanced light absorption, photocurrent, and efficiency in both liqud- and solid-state dye-sensitized solar cells using gold-silica core-shell nanoparticles [6].
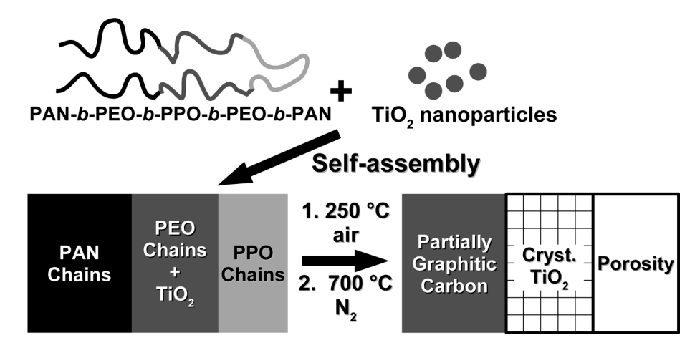
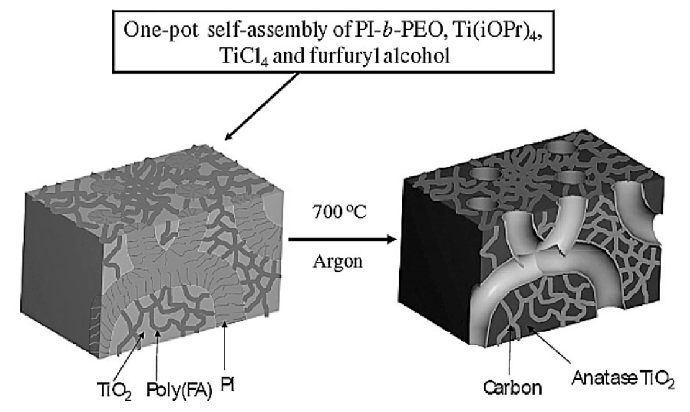
Other mesoporous materials we are currently exploring include titania/carbon composites using two different approaches. In our 2009 Macromolecules paper, we demonstrate the direct synthesis of mesoporous worm-like crystalline titanium dioxide covered with partially graphitic carbon [7]. These materials showed narrow, controllable pore size distributions through the self-assembly of an ABCBA block copolymer with added titanium dioxide sol. Titania/carbon composites have also been synthesized by mixing titanium dioxide sol and carbon precursors such as furfuryl alcohol and phenol/formaldehyde resols, with a diblock copolymer as a structure-directing agent. The detailed procedure has been reported in our 2011 Macromolecular Chemistry and Physics paper [7]. Furthermore, these composites with large cylindrical pores and narrow, controlled pore size distributions have been successfully demonstrated as anode materials for lithium ion batteries. Small crystallites of anatase titaniaare "wired" together with conductive carbon yielding small diffusion lengths. The devices showed good charge capacities at high rates and good cycle life, which could be due to the small grain size, allowing better accommodation of the volume change upon lithium insertion/desertion processes.
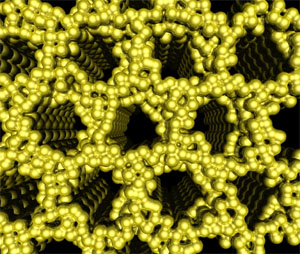
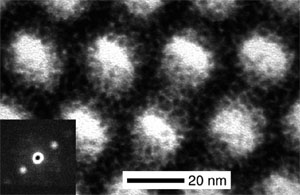
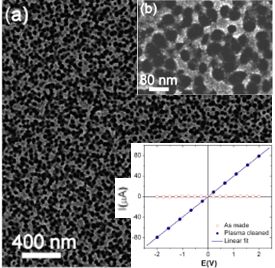
Finally, ordered mesostructured metals with tunable structures have gained widespread interest for application in energy conversion and storage. However, structure-directing metals into nanostructures have been challenging due to the high surface energy of metals, which favors lower surface area structures. With the development of particular charged ligand structures and the use of the CASH approach, we have recently succeeded in the synthesis of the first nanoporous Pt metal structures; this was achieved by structure-directing the functionalized nanoparticles with poly(isoprene)-b-poly(dimethylamino ethylmethacrylate) diblock copolymer in the bulk [8]. Various morphologies of the metal nanoparticle/block copolymer hybrids have been obtained by tuning the volume fractions, and the hybrids were disassembled in selective solvents to yield the nanoobjects [9]. Most recently, this approach has been extended to thin-film nanostructures on silicon substrate where metallic conductivity was observed for nanoporous Pt films [10]. Current research looks at applications for catalysis as well as generalizing the system for other metals and morphologies.
References
[1] Lee, J. et al. Direct Access to Thermally Stable and Highly Crystalline Mesoporous Transition-Metal Oxides with Uniform Pores. Nat. Mater. 7, 222-228 (2008).
[2] Orilall, M.C. et al. One-Pot Synthesis of Platinum-Based Nanoparticles Incorporated into Mesoporous Niobium Oxide-Carbon Composites for Fuel Cell Electrodes. J. Am. Chem. Soc. 131, 9389-9395 (2009).
[3] Crossland, E.J.W. et al. A Bicontinuous Double Gyroid Hybrid Solar Cell. Nano Lett. 9, 2807-2812 (2009).
[4] Docampo, P. et al. Control of Solid-State Dye-Sensitized Solar Cell Performance by Block-Copolymer-Directed TiO2 Synthesis. Adv. Funct. Mater. 20, 1787-1796 (2010).
[5] Nedelcu, M. et al. Monolithic Route to Efficient Dye-Sensitized Solar Cells Employing Diblock Copolymers for Mesoporous TiO2. J. Mater. Chem. 20, 1261 (2010).
[6] M. D. Brown, T. Suteewong, R.S. S. Kumar, V. D'innocenzo, A. Petrozza, M. M. Lee, U. Wiesner, H. J. Snaith. "Plasmonic Dye-Sensitized Solar Cells Using Core-Shell Metal-Insulator Nanoparticles", Nano Lett. 11 (2), 438-445 (2011).
[7] Stefik, M. et al. Three-Component Porous-Carbon-Titania Nanocomposites Through Self-Assembly of ABCBA Block Terpolymers with Titania Sols. Macromolecules 42, 6682-6687 (2009).
[8] Lee, J. et al. Direct Access to Mesoporous Crystalline TiO2/Carbon Composites with Large and Uniform Pores for Use as Anode Materials in Lithium Ion Batteries. Macromol. Chem. Phys. 212, 383-390 (2011).
[9] S. Warren, L. Messina, L. Slaughter, M. Kamperman, Q. Zhou, S. Gruner, F. DiSalvo, U. Wiesner. Science 320 (5884), 1748-1752 (2008).
[10] Z. Li, H. Sai, S. C. Warren, M. Kamperman, H. Arora, S. M. Gruner, U. Wiesner "Metal Nanoparticle-Block Copolymer Composite Assembly and Disassembly", Chem. Mater. 21 (23), 5578-5584 (2009).
[11] H. Arora, Z. Li, H. Sai, M. Kamperman, S. C. Warren, U. Wiesner, "Block Copolymer Directed Nanoporous Metal Thin Films", Macromol. Rapid. Commun. 31, 1960-1964 (2010).